BACKGROUND
Botox had a long history prior to Dr Adam Scott utiilising the purified form of Botulinum toxin in the 1970’s-80’s’s for an eye condition called strabismus. Studies were being performed as early as the 1820’s with speculations on the therapeutic value of the Botulinum Toxin. It was given the name the ‘Sausage Poison’ due to the reported deaths occurring as a result of eating blood sausage in the 1900’s. Later in the 1930’s researched focused on developing the toxin as an agent in warfare.
Botox has been used to treat a variety of conditions including blepharospasm, strabismus, cervi- cal dystonia, migraines, hyperhidrosis, and muscle spasticity.
In 2002 the FDA approved it’s use for cosmetic purposes. It is successfully used to treat the lines in the upper 1/3 of the face with more advanced techniques implemented when treating the lower third.
The injecting of Botulinum Toxin for the purpose of relaxation of facial muscles has become the most common non- surgical cosmetic procedure performed with predictable results and few, if any, side effects.
Research has spanned 2 decades investigating the safety, efficacy and complications regarding the use of Botox type A with continual advances being made.
The first study performed to investigate the utility Botox Type A on rhytides (wrinkles) or hyperfunctional facial lines was in the 1990’s.
WHAT IS BOTULINUM TOXIN (BOTOX) ?
The Botulinum Toxin is a neurotoxic protein produced by the bacterium Clostridium Botulinum that inhibits the release of acetylcholine at the neuromuscular junction causing temporary paralysis of the muscle smoothing the overlying skin, reducing wrinkles.
The effects of Botox are temporary and over time the neuromuscular signaling returns.
How are wrinkles formed?
Wrinkles are formed through a combination of repetitive contraction of facial muscles and dermal atrophy-thinning of the skin.
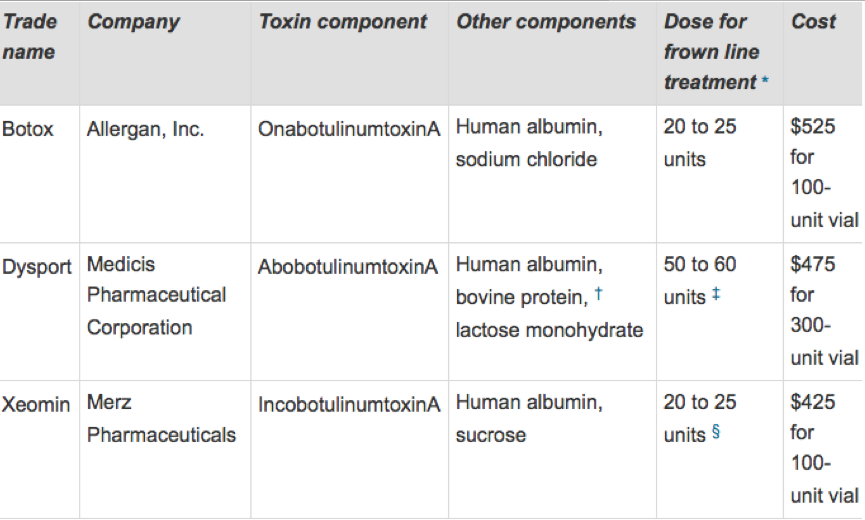
The clostridium bacterium produces a number of toxins labeled A-G with A being the most potent and appropriate for cosmetic purposes. There are 3 types of Botox Type A products being marketed which are approved for cosmetic purposes.
These are marketed under the trade names Botox, Xeomin and Dysport They are not interchangeable due to variation in formulation dosing and clinical response.
When studies comparing Botox to Dysport, the studies revealed Botox had a longer duration of effect and greater patient satisfaction than those receiving Dysport. (3 ) This may be due to the need to administer more Dysport to produce comparable effects to Botox (4)
Some suggestions have been made that Dysport provides greater diffusion into the muscle – meaning it’s able to have greater reach than the original site of injection.
There are no comparative studies between Xeomin and Botox but individual studies report the products to be similar in having comparable efficacy, duration of effect, and tolerability in healthy volunteers.
The effects of Botulinum Toxin Type A commonly last for three to six months, although there is a report of duration as long as twelve months. (1)
It appears that repeated treatments can result in a progressively longer duration of action. (2)(
A longitudinal study performed on twins over a period of 11 years suggest that long-term treatment can also result in additional benefits and prevent the formation of permanent lines. (5)
Assessing suitability
-
Dynamic wrinkles: wrinkles that are formed when laughing or squinting. These people demonstrate the most dramatic improvement.
-
Static wrinkles: wrinkles which are visible at rest are still candidates but results are slower.
-
Deep static lines may need a combination of dermal fillers and other cosmetic procedures for optimum results.
COMPLICATIONS
CONTRAINDICATIONS
HOW LONG DOES THE TREATMENT TAKE ?
After the initial consultation and depending on the areas being treated the time may range between 20 -30 mins. There may be some swelling, redness or bruising around the site of injection .
FINALLY
There are a lot of studies supporting the use of Botulinum Toxin type A in facial rejuvenation when administered correctly and limited to the use as indicated by the FDA and TGA guidelines in the USA and Australia.
References
1/ Carruthers A, Carruthers J, Said S. (2005) Dose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in females. Dermatol Surg. 2;31(4):414–422. [PubMed]
2/ CarruthersJA,LoweNJ,MenterMA,etal.(2002)Amulticenter,double-blind,random- ized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 46:840-849.
3/ Nicholas J. Lowe, MD. (2007) Overview of Botulinum neurotoxins.Journal of Cosmetic and Laser Therapy.2007;9(suppl 1):11-16 Retrieved from ebscohost.com jan14/1/2015
4/ Lowe P, Patnaik R, Lowe N.(2006) Comparison of two formulations of botulinum type A for the treatment of glabellar lines: a double-blind, randomized study. J Am Acad Dermatol. ;55:975–80. Retrieved from ebscohost.com jan14/1/2015
5/ William J.Binder, MD( 2006) Long-Term Effects of Botulinum Toxin Type A(Botox),A Comparison in Identical Twins, ARCH FACIAL PLAST SURG/VOL 8, NOV/DEC
retrieved from http://archfaci.jamanetwork.com/ by a Queensland Health User on 01/04/2015